The possibility of genuinely experiencing multiple stimuli, thoughts or affective states simultaneously is a contentious topic. While one view holds that it is possible to multitask and attend to multiple stimuli in a parallel manner, another holds that what one may feel as attention to multiple stimuli in fact involves switching among them very rapidly, in a serial manner [1]. The latter view is consistent with theories that compare conscious awareness to a spotlight in a dark room, where the spotlight represents the focus of one’s awareness, and the dark room encompasses all sensory inputs, perceptions, memories, and affective states immediately available. Like a moving flashlight, it illuminates one experience at a time [2,3].
This scientific debate holds significance for assessments of animal welfare, as assumptions about how concurrent affective states (positive or negative) permit, exclude, or modulate one another can critically affect estimates of the actual times experienced in the affective states examined. This is especially true if we consider that organisms may frequently experience welfare challenges that overlap in time. Here we explore the role of attention on affective experiences within the context of ‘Cumulative Pain’ assessments [4] (where pain is used as a shorthand for any negative affective experiences).
For instance, consider a hypothetical scenario where an individual is exposed, in one day, to two consecutive sources of Hurtful pain for five hours each. In this case, Cumulative time in pain would be represented by 10 hours. But if the two pain episodes unfolded simultaneously, would Cumulative Pain still be best represented by 10 hours? And during the five hours of simultaneous exposure to these two pain sources, would there be any time left for positive experiences? Would this change if the second source of pain was of a different intensity (e.g., Annoying)?
So far, estimates of Cumulative Pain have been based on the operational assumption that multiple concurrent challenges have the above mentioned additive effect on experience (namely that their effects when experienced simultaneously are best represented by the sum of their isolated effects). This solution works well for the purpose of distinguishing the impact of welfare challenges across scenarios or production systems [5,6]. However, to the extent that attention is selective, switching among simultaneously occurring experiences, estimates of cumulative time in pain can be refined to incorporate this phenomenon. Such a refinement should enable estimating the time individuals are aware of and focused on each negative experience (as opposed to simply being exposed to them). Additionally, it allows exploring the time that remains available for individuals to enjoy positive affective experiences, once the time actually preoccupied with ‘pain’ is properly discounted.
ATTENTION AS A LIMITED RESOURCE
The dynamic nature of attention plays a critical role in our affective experiences. Our affective states are not static, instead fluctuating as our attention shifts between various aspects of a situation, memory, or sensory input. This continuous shift in attention leads to the processing and prioritization of different emotions or emotional cues at different points in time.
The involuntary capture of attention by pain is a crucial aspect of its role as a warning signal of (real or perceived) danger. Pain demands attention, as it must change behavior to prevent survival and reproduction from being compromised [7–10]. In general, the greater the threat, the more intense the pain signal [11,12], engaging more of the individual’s attention [10,12–15].
Attention is, however, a limited resource, constrained by the brain’s architecture, its finite capacity for neural processing and limited supply of energy. Amidst the constant influx of vast amounts of sensory data, various mechanisms evolved to direct the brain’s attention to relevant information, while suppressing less relevant stimuli. For example, top-down mechanisms of attentional control are driven by an individual’s goals and expectations (e.g., if an individual is searching for red food, these mechanisms will enhance the processing of red stimuli while suppressing other colors) [16,17]. Allocation of attention is also determined by bottom-up saliency mechanisms that are triggered by the inherent features of stimuli, particularly their intensity, novelty, or contrast [18]. Sensory processing areas respond more robustly to more intense, contrasting and novel stimuli, and this enhanced activity increases the likelihood that these stimuli are attended to [19].
The perception of pain, both physical and psychological, is closely intertwined with these attentional processes. Pain’s ability to capture attention is also largely mediated by bottom-up saliency mechanisms triggered by the inherent features of the painful experience, such as their intensity or sudden onset. For example, in the case of physical pain, as intensity increases, nociceptors transmit more frequent and larger electrical signals along the pain-processing pathway, triggering the release of greater amounts of neurotransmitters like glutamate (a primary excitatory neurotransmitter) and neuromodulators like substance P (involved in pain transmission and sensitization of nociceptors). This, in turn, intensifies the activation of pain-processing neurons and amplifies pain signals within the neural network, capturing more attention to ensure that organisms respond adequately [20,21] .
Pain is also modulated by voluntary mechanisms such as attentional focus [22]. Consciously directing attention towards a painful stimulus can increase the perception of pain intensity, given the heightened awareness of the pain sensation and the amplification of emotional components of pain, such as anxiety or distress [23,24]. Conversely (and this is also of great relevance to strategies to improve animal welfare), reduced pain is observed if attention is directed away from the painful stimuli by more salient experiences (e.g., fear, stress, highly motivated tasks) [21]. By occupying the brain’s limited attentional resources with alternative tasks or stimuli, the processing of pain signals is inhibited, leading to a reduction in pain perception.
TIME IN CONCURRENT AFFECTIVE STATES OF DIFFERENT INTENSITY
A natural conclusion from the previous section is that when individuals are exposed to simultaneous pain sources that differ in their salience, the brain will prioritize processing of the more salient one (i.e., more intense, novel or threatening), devoting a greater share of its limited attentional resources.
Top-down mechanisms can also lead to inhibition of concurrent, less salient, pain. One such mechanism is known as conditioned pain modulation (CPM), or ‘diffuse noxious inhibitory control’ (a term used more often in animal research), sometimes described as ‘pain inhibits pain’ [25]. CPM occurs when response from a painful stimulus is inhibited by another, often spatially distant noxious stimulus. The process involves the activation of descending inhibitory pathways that can reduce the activity of pain-transmitting neurons in the spinal cord. One means whereby this modulation happens is through the release of endogenous opioids, which inhibit the release of neurotransmitters (e.g., substance P, glutamate) needed for pain transmission. Engagement in pleasant and highly motivated activities and movement may also trigger the release of endogenous opioids, as well as other neurochemicals like dopamine, serotonin, and oxytocin, which can too contribute to a reduced perception of pain. Additionally, a strong painful experience may also serve as an anchor or comparison point by which others are judged (hence reducing their intensity relative to when they are judged individually) [26]
If less intense pain is nearly overshadowed by more intense pain, then the experience of simultaneous pain cannot be represented by the sum of the experience of each pain stimulus when experienced alone. High intensity pain will likely eliminate less intense pain during those moments when attention is recruited to the former.
For the purposes of Cumulative Pain analysis, and until further knowledge is available, we therefore assume that when an individual experiences pain episodes of different intensities concurrently, only the most intense pain should be computed.
TIME IN NEGATIVE AFFECTIVE STATES OF SIMILAR INTENSITY
With concurrent pain of similar intensity a more nuanced strategy is required. In line with observations that increasing the area of exposure to noxious stimuli produces a disproportionately small (sub-additive) increase in the perceived intensity of pain [27], we consider the possibility that for pain of similar intensity, the share of attention devoted to the experience is summated in a sub-additive way. The extent to which summation is sub-additive is, however, unclear. As a starting point for further investigation and debate, we consider two scenarios:
Strategy A: conditional sub-additivity that depends on pain intensity
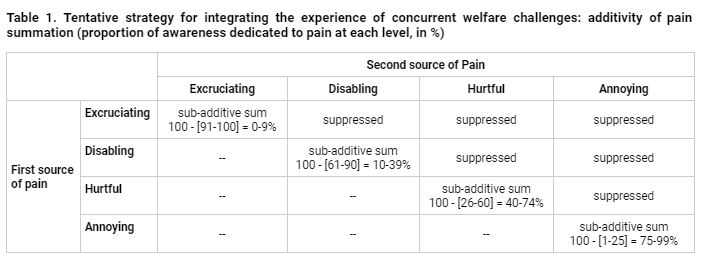
Because pain of greater intensities demands a greater share of the organism’s attention, remaining attentional resources for the processing of additional pain would become progressively scarcer. A tentative set of estimates for the degree of attention allocated to each intensity category is as follows: (1) Excruciating: 91-100%, (2) Disabling: 61-90%, (3) Hurtful: 26-60%, (4) Annoying: 1-25%. In Table 1, these estimates are used to suggest the extent to which sub-additivity is expected with simultaneous sources of similarly intense pain in each case.
For example, in the case of Excruciating pain, these thresholds would suggest that when a second source of Excruciating pain is experienced simultaneously, the maximum increase in the time attending to the new source of pain (in addition to that grabbed by an isolated source of Excruciating pain) would be 9%. Conversely, if two sources of Annoying pain are experienced simultaneously, there would be near complete additivity (70-99%).